PRI’s Dr. Amy Grooms talks about the SAINT neuromodulation system in a recent interview on KTHV-TV, Channel 11.
The UAMS Psychiatric Research Institute’s Interventional Psychiatry program offers pioneering treatments for depression, bipolar disorder and other mental health conditions. These treatments are FDA-approved and very effective in helping individuals recover from their conditions, particularly if these conditions have not improved from the use of antidepressants or psychotherapy.
To find out more about the Interventional Psychiatry Program, call 501-526-8650 or email us at psychiatry-interventional@uams.edu. The program will accept referrals from a patient’s current psychiatrist by faxing a recent history to 501-603-1897 to the attention of Interventional Psychiatry.
Here are some of the treatments offered by the program:
SAINT®
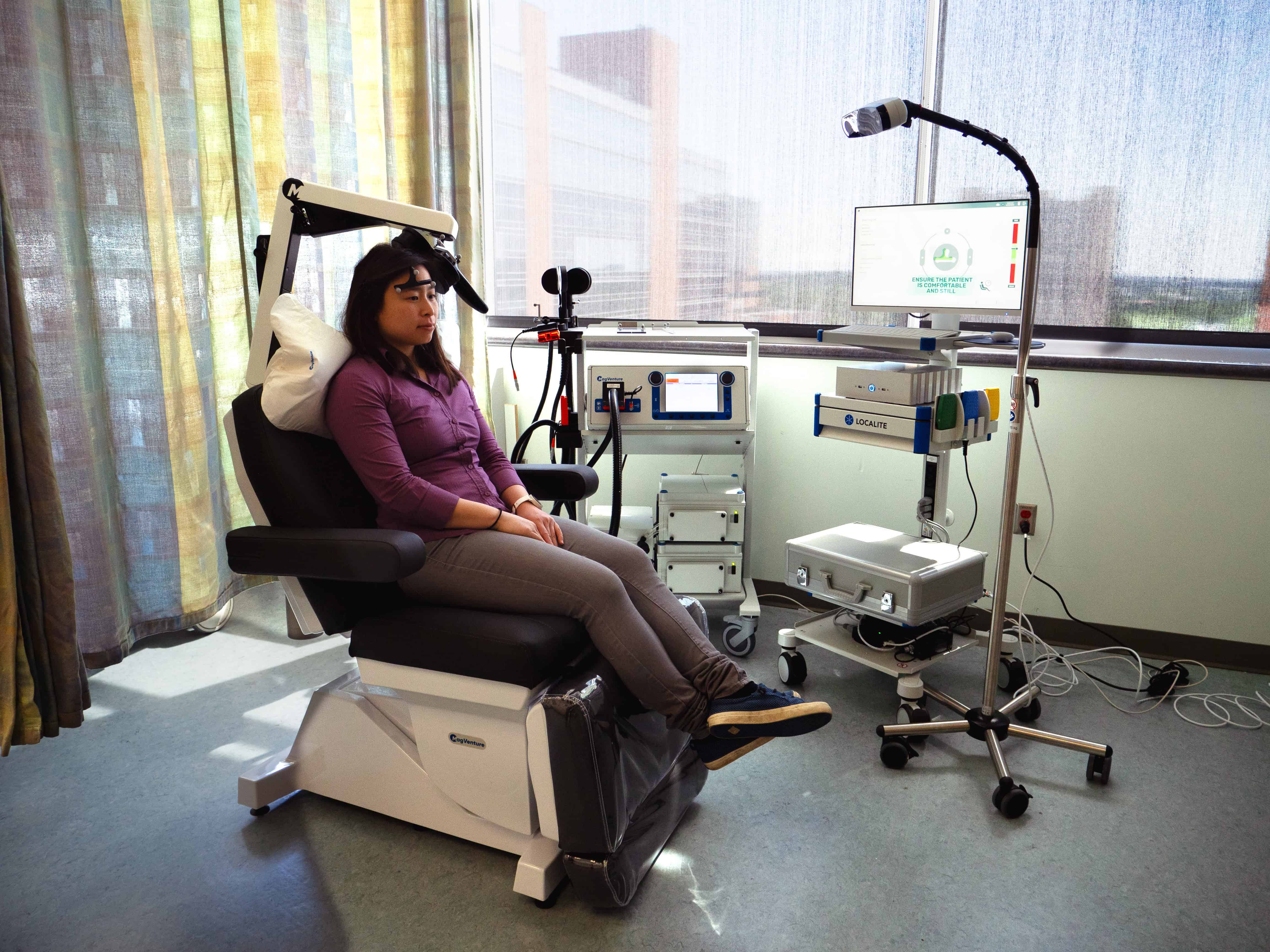
UAMS’ Psychiatric Research Institute (PRI) is the first facility in the nation to partner with Magnus Medical, Inc., to implement the SAINT® neuromodulation system, an innovative, one-of-a-kind therapy for treatment-resistant depression. The SAINT system uses magnetic resonance imaging (MRI) to pinpoint the optimal anatomical target for precise transcranial magnetic stimulation (TMS) in individuals with major depression. The targeting process helps identify the location and correct stimulation dosing for each patient. A specialized pattern of magnetic pulses is then delivered to cause neurons to fire and rebuild connectivity.
The SAINT treatment protocol at PRI relies on the unique capabilities of the Helen L. Porter and James T. Dyke Brain Imaging Research Center’s MRI scanner. The MRI scan, which takes approximately 45 minutes, will occur prior to treatment. Using the MRI scan, the individual patient’s optimal treatment target is mapped by Magnus’ technology. SAINT treatment consists of 10 sessions per day for five consecutive days. Each session includes 10 minutes of stimulation followed by a 50-minute rest period. Treatment is non-invasive, and the most common side effect is headache.
Initially, patients from PRI’s inpatient unit will be treated with the SAINT neuromodulation system, which received Food & Drug Administration approval in 2022. Patients can potentially start experiencing symptom relief within days (versus weeks when standard TMS protocols are used). In a double-blinded randomized controlled trial (RCT) evaluating SAINT, 79 percent of people in the active treatment group experienced remission of their depression symptoms following the five-day treatment protocol, compared to people in the control group (13 percent remission rate) who received a “sham” (placebo) treatment.
Patient referrals should be faxed to 501-603-1897. Call 501-526-8650 to set up a consultation.
Electroconvulsive therapy
Electroconvulsive therapy is used for patients with severe mood disorders (depression and bipolar) that have not responded to other forms of treatment. When a disorder becomes this severe, it is referred to as treatment-resistant depression. Electroconvulsive therapy is a primary a treatment choice for treatment-resistant depression, psychotic depression and acute suicidality. Also, it can be an ideal choice for severe depression during pregnancy and afterwards (postpartum depression), thereby allowing minimal exposure of the baby to medications.
Electroconvulsive therapy involves brief electrical stimulation of the brain conducted while the patient is under anesthesia. The electroconvulsive therapy team of medical professionals at the Psychiatric Research Institute includes a psychiatrist, an anesthesiologist and a nurse, all highly trained and experienced.
Ketamine
Ketamine has been used primarily as an anesthetic since the 1960s. Recently, it has been shown in some individuals to dramatically improve the symptoms of patients with severe depression, especially those with suicidal thoughts. The Psychiatric Research Institute is currently using two forms of this drug to treat suicidal ideation and treatment resistant depression:
Looking for more information?
Complete the form below and hit the Submit button and we will be in touch!